Regulatory and ANVISA: BizaLumière as a Strategic Partner in Analytical Documentation and Laboratory Quality
REGULATÓRIO E ANVISA
9/30/20253 min read


Pharmaceutical regulation in Brazil is one of the pillars that ensures the safety, efficacy, and quality of medicines made available to the population. ANVISA (National Health Surveillance Agency) plays a key role in this process, involving complex technical evaluations that demand precise documentation, reliable data, and robust scientific justification.
In this context, BizaLumière emerges as a strategic partner for pharmaceutical companies, offering specialized technical consulting that goes far beyond compliance: we help build solid foundations for safe regulatory decisions and consistent laboratory processes.
Regulatory Landscape and the Importance of Compliance
Regulatory compliance is not just a legal obligation — it’s a competitive advantage. Companies with structured processes can:
Respond quickly to ANVISA’s requirements
Reduce risks of rejection and rework
Ensure predictability in product launches and submissions
Reinforce their reputation for quality and reliability
Without proper alignment, consequences may include delays, additional costs, reformulations, and even loss of commercial opportunities and international partnerships.
Critical Points Required by ANVISA
Consistency of analytical data
Clarity in technical justifications
Adequacy of supporting documents
Traceability of all documented stages
Key ANVISA Regulations and Guidelines
ANVISA aligns with global standards, adopting the CTD (Common Technical Document) as an international reference for submissions. Key regulations include:
RDC 200/2017 – Registration of new medicines
RDC 73/2016 – Post-registration changes
RDC 359/2020 – Rules for CADIFA and DMF of active pharmaceutical ingredients
These regulations define clear criteria for dossier presentation, stability requirements, impurity control, and packaging compatibility.
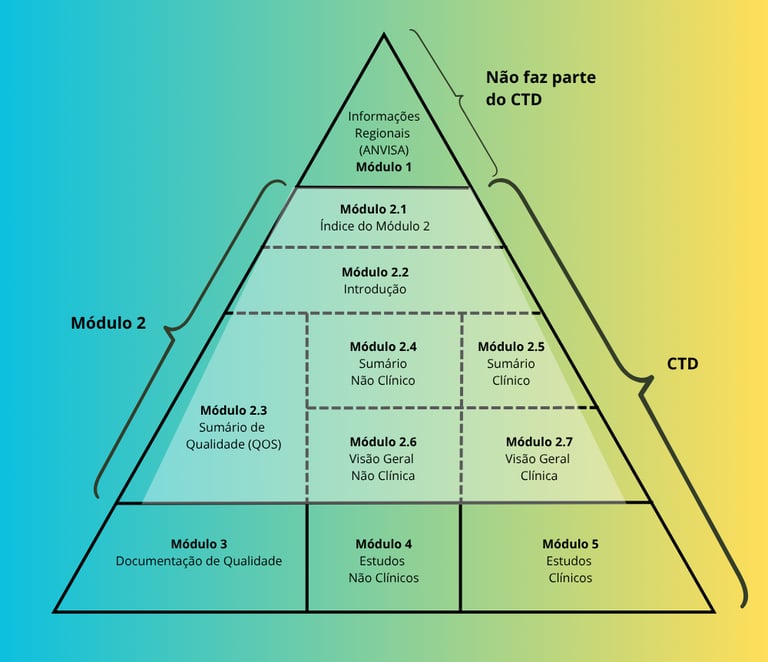
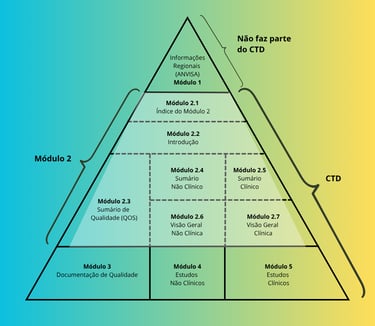
Technical Submissions: CTD Structure
The CTD format organizes technical and regulatory data into modules, streamlining ANVISA’s review process and aligning national practices with international standards.
Module 2 (Technical Summaries): Summarizes quality, efficacy, and safety with clarity and objectivity
Module 3 (Quality): Presents analytical and pharmaceutical data, including specifications, validation methods, and stability studies
A well-structured dossier ensures clarity, consistency, and regulatory alignment, reducing rework and strengthening submission credibility.


How BizaLumière Supports Your Company in Practice
BizaLumière offers specialized technical consulting in:
Drafting and reviewing analytical documents (methods, SOPs, URS, specifications)
Preparing CTD dossiers (Modules 2 and 3)
Responding to ANVISA’s technical requirements with clarity and scientific grounding
Investigating OOS/OOT, deviations, and CAPA using Ishikawa, 5W2H, and FMEA
Evaluating impurities and residual solvents with ICH-based justifications
Conducting stability and forced degradation studies to define shelf life
Analyzing drug-packaging compatibility to ensure physicochemical integrity
Critically reviewing DMF and CADIFA to ensure traceability and compliance of inputs
Reviewing certificates and reports (CoA, GMP, technical)
Training teams in Good Analytical Practices (GAP)
Managing documentation and data integrity
Regulatory Responses and Post-Submission Support
After initial submission, ANVISA may request additional information. BizaLumière supports your team in crafting well-founded technical responses with:
Logical organization and clear communication
Consistent supporting documents (technical reports, certificates, lab results)
Integration of analytical, pharmaceutical, and regulatory data
Scientific justification for critical parameters such as purity, potency, and stability
This approach reduces rework risks and increases the likelihood of acceptance by the agency.
Input Management and Supporting Documentation
Regulatory compliance also depends on input quality and document traceability. We assist in:
Critical evaluation of DMF (Drug Master File): synthesis, specifications, methods, and stability
Technical analysis of CADIFA: verifying process consistency and pharmacopeial compliance
Thorough review of certificates (CoA, GMP, technical reports): authenticity, validity, and regulatory adherence
Trends and Challenges in the Regulatory Landscape
Brazil’s regulatory sector follows global trends:
Trend / Market Impact
Digitization / Faster submissions and improved traceability
International harmonization / Facilitates partnerships and global competitiveness
Demand for robust data / Requires greater technical investment
Focus on inputs (CADIFA/DMF) / Ensures safety and quality throughout the supply chain
Stability studies, drug-packaging compatibility, and robust documentation are increasingly valued by ANVISA, requiring technical consistency and practical applicability.
BizaLumière’s Differentials
Technical consulting, not auditing: focused on real-world applicability
Specialized team in analytical documents that support regulatory decisions
Clarity, consistency, and standardized scientific language
Personalized service and post-submission follow-up
Commitment to deadlines and the pharmaceutical industry’s routine
Conclusion: Your Technical Foundation with BizaLumière
At BizaLumière, we understand that regulatory quality is built on solid foundations of documentation, reliable data, and well-justified technical decisions.
Beyond compliance, we offer regulatory intelligence and analytical structure to turn challenges into practical solutions.
👉 Contact us to learn how we can support your team with true technical consulting.
BizaLumière
Specialized consulting in Technical Documentation and Analytical Regulatory Requirements
ContaCT
newsletter
© 2025. BizaLumière. All rights reserved.

Privacy Policy
